Table of Contents
ToggleIn the U.S. Life Sciences sector, managing interactions with Healthcare Professionals (HCPs) and Healthcare Organizations (HCOs) is critical for maintaining compliance with regulatory standards and ensuring ethical business practices.
The rise of data-driven HCP engagement solutions offers a transformative approach to these interactions, providing robust frameworks for transparency, accountability, and efficiency.
This blog delves into the compliance fundamentals of HCP engagements, emphasizing the role of a data-driven approach in enhancing compliance through integrated platforms, trend analysis, and comprehensive audit trails. We will also outline seven key steps for compliant HCP engagements, providing best practices to optimize strategies while maintaining transparency and trust.
Compliance Fundamentals in HCP Engagements
Transparency Reporting
Transparency in HCP engagements is not just a regulatory requirement but a cornerstone of ethical practice. The Physician Payments Sunshine Act mandates that manufacturers of drugs, medical devices, and biologics covered by Medicare, Medicaid, and the Children’s Health Insurance Program (CHIP) report payments and other transfers of value to HCPs and HCOs. This requirement promotes accountability and allows the public to understand the financial relationships between HCPs and life sciences companies.
Regulatory Standards
The compliance landscape is governed by various federal and state regulations aimed at preventing fraud and abuse, ensuring patient safety, and maintaining ethical standards. Key regulations include the Anti-Kickback Statute (AKS), the False Claims Act (FCA), the Foreign Corrupt Practices Act (FCPA), and the Sunshine Act. Adhering to these regulations is essential for avoiding hefty fines, legal consequences, and reputational damage.
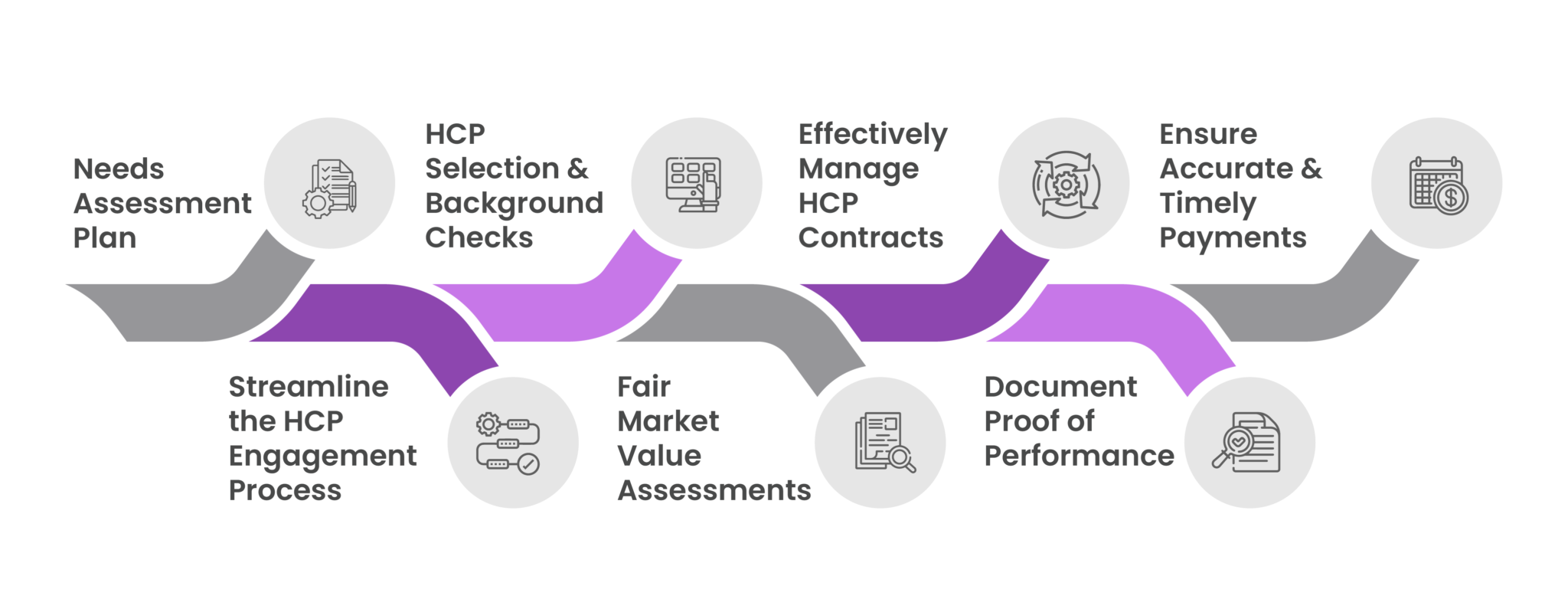
Key Steps for Compliant HCP Engagements
1. Needs Assessment Plan
- Utilize Automation to Assess Business Needs
Automation tools can streamline the needs assessment process, ensuring that HCP engagements are strategically aligned with organizational objectives. By automating data collection and analysis, companies can identify specific business needs and plan engagements that fulfill these requirements while maintaining compliance.
- Systematic Budgeting Process
A systematic budgeting process is essential for tracking services provided and aligning expenditures with predefined business needs. Automation can facilitate this process, providing real-time visibility into budget allocations and expenditures, and ensuring that they are consistent with compliance standards.
2. Streamline the HCP Engagement Process
- Ensure Consistency Across Departments
Consistency is key to a compliant HCP engagement process. This requires collaboration across multiple departments such as compliance, marketing, medical affairs, and others. An integrated platform can facilitate communication and coordination, ensuring that all stakeholders are aligned.
- Develop a Holistic View of Engagements
A holistic view of HCP engagements ensures control and compliance throughout the engagement lifecycle. Data-driven HCP Engagement solutions can provide comprehensive dashboards and reports, offering insights into the status of engagements, compliance checks, and potential issues.
3. HCP Selection and Background Checks
- Centralized Database for HCP Selection
Establishing a centralized database for HCP selection streamlines and standardizes the process. This database should include criteria for selection, ensuring that chosen HCPs meet the necessary qualifications and compliance requirements.
- Automated Screening of HCPs
Automated screening of healthcare professionals against multiple databases such as the Office of Inspector General (OIG), System for Award Management (SAM), and the Food and Drug Administration (FDA) can significantly reduce the time required to ensure compliant engagements with qualified healthcare professionals.
- Consistent Information Across Departments
A centralized database system ensures that all departments have access to consistent information regarding HCP qualifications. This uniformity promotes a standardized approach to HCP selection and reduces the risk of non-compliance.
4. Fair Market Value Assessments
- Algorithms for Fair Market Values
Integrating algorithms to determine fair market values (FMVs) based on geographic locations and tiers ensures compliance with approved compensation rates for HCP engagements. These algorithms should be regularly updated to reflect current market conditions.
- Automation in Compensation Validation
Automation can assess and validate the usage of approved compensation rates consistently throughout the organization. This ensures that all payments to HCPs are fair, justified, and compliant with regulatory standards.
5. Effectively Manage HCP Contracts
- Centralized Contract Storage
Utilizing a platform with integration for centralized storage of executed contracts simplifies the management process. This system should be capable of integrating with a legal contract management system, ensuring that all contracts are accessible and compliant.
- Automation in Contract Management
Automation streamlines the contract management process, from drafting and reviewing to execution and storage. Automated workflows ensure that all contracts go through the necessary compliance checks and approvals.
- Linking Engagement Requests to Contracts
Creating a process that links actual HCP engagement requests with the appropriate contracts ensures that all engagements are covered by valid, compliant agreements. This linkage provides a clear audit trail and simplifies compliance monitoring
6. Document Proof of Performance
- Comprehensive Documentation of Activities
Comprehensively compiling proof of performance is essential to demonstrate that the activities for which healthcare professionals (HCPs) were contracted were completed before payment, ensuring compliance. This includes monitoring and documenting changes in FMV rates, HCP contracts, and more.
- Final Compliance Check
Conducting a final compliance check ensures that all HCP activities adhere to regulatory requirements and internal policies. This step verifies that all necessary documentation is in place and that the engagements meet compliance standards.
7. Ensure Accurate & Timely Payments
- Effective Payment Controls
Implementing effective payment controls ties with the accounts payable system to substantiate proper and timely payments to HCPs. Automation can facilitate this process, ensuring that payments are accurate, timely, and compliant.
The Role of Data-Driven HCP Engagements
Data-driven platforms are essential for effective HCP engagement, and qordata’s Global HCP Engagement Solution offers a powerful suite of features to streamline this process.
Integrated Platforms for Tracking Interactions:
Consolidated Data: qordata’s solution integrates with various systems, including Veeva and other CRMs and contract management software. This eliminates data silos and provides a unified view of all HCP interactions, including expenses, payments, and communication.
Enhanced Compliance Monitoring: Real-time data facilitates efficient monitoring of interactions. qordata’s built-in compliance checks ensure adherence to internal policies and regulations.
Data-Driven Analysis for Informed Decisions:
Advanced Analytics: qordata’s platform goes beyond basic tracking. Its analytics capabilities enable you to identify trends and patterns in HCP engagement data. This allows you to gain a deeper understanding of what resonates with HCPs, optimizing your engagement strategies for better results.
Proactive Risk Management: Identify potential compliance risks before they become problems, allowing for proactive mitigation strategies.
Targeted Improvements: Leverage data to pinpoint areas for improvement in your HCP engagement efforts.
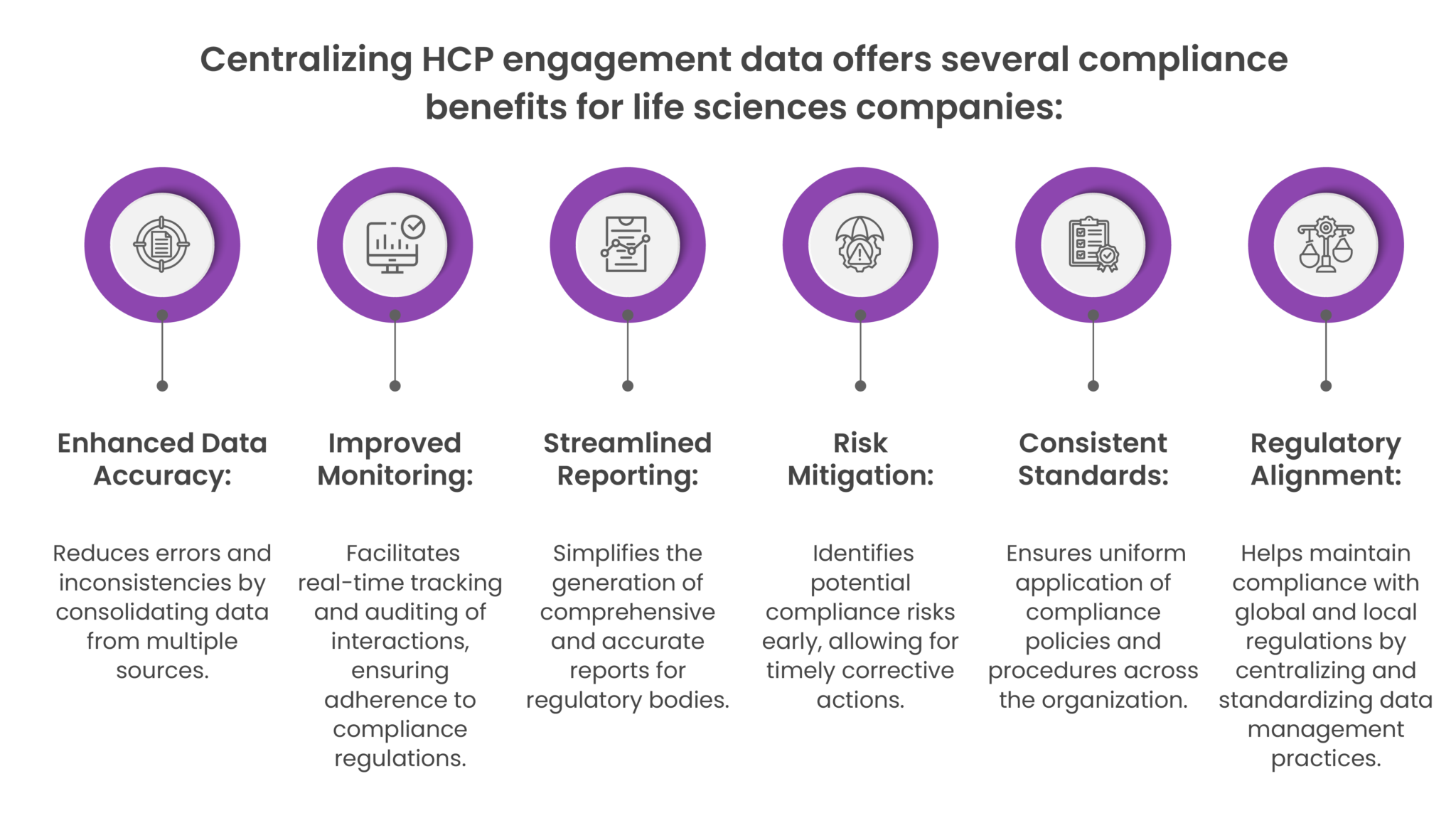
Maintaining Audit Trails for Transparency:
Comprehensive Records: qordata ensures a complete audit trail for every HCP interaction. This includes documentation of expenses, payments, communication, and debarment screening details.
Regulatory Compliance: Having a transparent and readily available audit trail simplifies internal reviews and external regulatory audits. qordata’s system demonstrates your commitment to compliance standards.
Conclusion
In the dynamic and highly regulated U.S. Life Sciences sector, compliant HCP engagements are crucial for maintaining ethical standards, ensuring transparency, and avoiding regulatory penalties. Data-driven solutions offer a powerful approach to managing these engagements, providing tools for tracking, analyzing, and documenting interactions with HCPs.
By following the seven key steps outlined in this blog, healthcare companies can optimize their HCP engagement strategies, ensuring compliance and fostering trust with stakeholders. Embracing these data-driven methodologies not only enhances compliance but also drives efficiency and effectiveness in HCP interactions, ultimately contributing to better patient outcomes and advancing the mission of life sciences organizations.
Other Relevant Reads: